
Why the crystal structure of the element is such lattice but not another?
Категория реферата: Топики по английскому языку
Теги реферата: сочинение ревизор, новейшие рефераты
Добавил(а) на сайт: Chirkash.
Предыдущая страница реферата | 12 13 14 15 16 17 18 19 20 21 22 | Следующая страница реферата
We tried to unravel the puzzle, but instead we received a new puzzle which provides a good explanation for the physico-chemical properties of the elements. This is the “coordination number” 9 (nine) for the face-centered and volume-centered lattices.
This frequent occurrence of the number 9 in the table suggests that the densest packings have been studied insufficiently.
Using the method of inverse reading from experimental values for the uniform compression towards the theoretical calculations and the formulae of Arkshoft and Mermin (1) to determine the Z value, we can verify its good agreement with the data listed in Table 1.
The metallic bond seems to be due to both socialized electrons and “valency” ones – the electrons of the atomic kernel.
Список литературы
Solid state physics. N.W. Ashcroft, N.D. Mermin. Cornell University, 1975
Characteristics of elements. G.V. Samsonov. Moscow, 1976
Grundzuge der Anorganischen Kristallchemie. Von. Dr. Heinz Krebs. Universitat Stuttgart, 1968
Physics of metals. Y.G. Dorfman, I.K. Kikoin. Leningrad, 1933
What affects crystals characteristics. G.G.Skidelsky.’’ Engineer’’ № 8, 1989,Moscow.
Introduction into physical chemistry and chrystal chemistry of semi-conductors. B.F. Ormont. Moscow, 1968.
Appendix 1
Metallic Bond in Densest Packing (Volume-centered and face-centered)
It follows from the speculations on the number of direct bonds ( or pseudobonds, since there is a conductivity zone between the neighbouring metal atoms) being equal to nine according to the number of external electrons of the atomic kernel for densest packings that similar to body-centered lattice (eight neighbouring atoms in the first coordination sphere). Volume-centered and face-centered lattices in the first coordination sphere should have nine atoms whereas we actually have 12 ones. But the presence of nine neighbouring atoms, bound to any central atom has indirectly been confirmed by the experimental data of Hall and the uniform compression modulus (and from the experiments on the Gaase van Alfen effect the oscillation number is a multiple of nine.
Consequently, differences from other atoms in the coordination sphere should presumably be sought among three atoms out of 6 atoms located in the hexagon. Fig.1,1. d, e shows coordination spheres in the densest hexagonal and cubic packings.
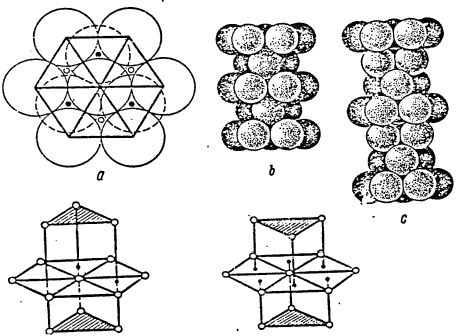
Fig.1.1. Dense Packing.
It should be noted that in the hexagonal packing, the triangles of upper and lower bases are unindirectional, whereas in the hexagonal packing they are not unindirectional.
Appendix 2
Theoretical calculation of the uniform compression modulus (B).
B = (6,13/(rs|ao))5* 1010 dyne/cm2
Where B is the uniform compression modulus
аo is the Bohr radius
rs – the radius of the sphere with the volume being equal to the volume falling at one conductivity electron.
rs = (3/4 pn ) 1/3
Where n is the density of conductivity electrons.
Table 1. Calculation according to Ashcroft and Mermin
|
Element Рекомендуем скачать другие рефераты по теме: детские рефераты, титульный курсовой работы, сочинение татьяна. Предыдущая страница реферата | 12 13 14 15 16 17 18 19 20 21 22 | Следующая страница реферата Поделитесь этой записью или добавьте в закладкиКатегории: |